The influence of the Brazil nut effect on particle measurement
Correlation of representative sampling and quality of the measurement result
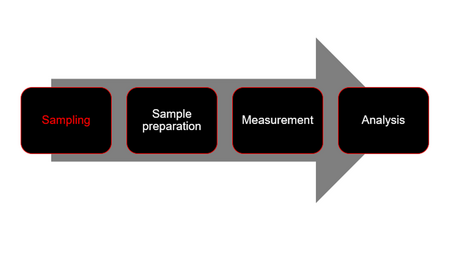
An often-neglected step in sample analysis is sampling (Figure 1). The relevance of correctly performed (representative) sampling is based on the principles of good laboratory practice (GLP) as well as international standards, such as DIN EN ISO/IEC 17025 (standard for laboratory accreditation in the field of testing and calibration). After all, what is the use of a measurement that is correct in itself if the sample is not representative with regard to its basic population and thus reliability could not be guaranteed? Particularly when determining particle size distributions, e.g. by means of static light scattering, sampling is a decisive factor for the success of the measurement.
The importance of representative sampling
The necessity of sampling results from the discrepancy between the population of a sample and the sample quantity required for laboratory analysis. In the field of static light scattering, this ranges from 1 mg to about 100 g, depending on the material and particle size. In the chemical industry in particular, it is therefore necessary in extreme cases to infer from a spatula tip of analysis material the particle size distribution of a total of several kilograms to tons of starting material. Solid samples in particular do not usually have a homogeneous distribution, which results in variances in the analysis. This applies in terms of their physical or chemical properties and in terms of their particle size distribution:
• Temporal changes of sample properties: Inhomogeneous distributions of the sample can be exacerbated if, for example, poor storage conditions (fluctuating ambient conditions and/or inadequate storage containers) lead to changes in sample properties over time.
• "Wrong place of sampling": The sample is usually taken only from the top or from easily accessible locations instead of considering the entire sample volume. Due to inertia, convection or buoyancy, segregation of the sample can occur. For example, transporting samples can cause the so-called Brazil nut effect to occur: Depending on the density, shape, surface properties, orientation and external influences (e.g. kinetic energy), cavities are created for a short time which are occupied by small particles. Since the larger particles cannot occupy these cavities, they virtually migrate upward (Figure 2). The inverse Brazil nut effect, i.e. the settling of the large particles towards the bottom of the sample, is also observed with suitable parameters.
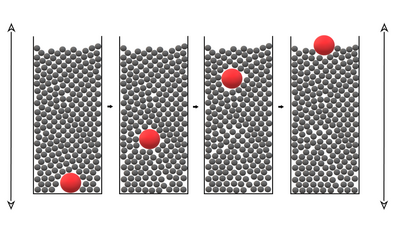
Figure 2: In the Brazil nut effect, large particles migrate upward due to external influences.
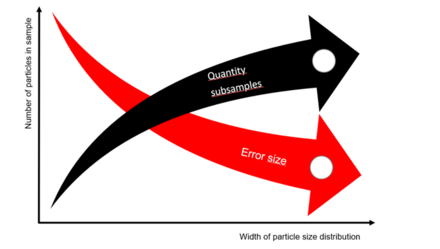
The goal of correct sampling is to minimize analysis variances. However, even well-mixed samples are subject to statistical variation. The confidence interval, i.e. the area around the mean value in which the sought true value is located with a given probability, can only be optimized by increasing the number of analyzed subsamples. The size of the statistical error of the sampling depends mainly on the sample size and the width of the particle size distribution. The fewer particles the sample contains and the wider the particle size distribution, the larger the error can become. Therefore, the number of characterized subsamples should increase with increasing heterogeneity of the total sample (Figure 3).
Options for sample division for particle measurement
For sample division, the amount of sample needed for analysis should first be determined. The amount of sample needed depends on the analytical method itself and the particle characteristics being analyzed. Once the required sample amount is determined, the laboratory sample must be thoroughly mixed and divided until the amount needed for analysis is obtained. Commonly used methods for sample division include coning and quartering, the use of a riffle divider, or the use of a rotary cone sample divider (Figure 4). For very large sample volumes, as an alternative to sample splitting, several randomly distributed samples can be taken from the total volume and combined into one analytical sample. In both sample division and sampling, care must be taken to ensure that no particle class is over-represented.
If the sample quantity of a solid sample is not sufficient for division, it can be converted into a suspension by dispersing it in a suitable liquid. It is essential to ensure that the mixture does not react either physically or chemically. Following thorough mixing, sampling is then carried out with a pipette a few centimeters above the bottom of the vessel in the case of homogeneous suspensions. In the case of inhomogeneous suspensions or rapidly sedimenting samples, the entire suspension must be analyzed, as otherwise widely differing results may be obtained with different analysis samples. Alternatively, for samples with large particles or agglomerates, a paste can be prepared by first adding a few drops of the dispersing medium to the feed material and then carefully mixing it with a spatula or glass rod. Sampling can then be carried out, for example, with a glass thread.
It should be noted that in the case of highly inhomogeneous samples, which do not provide consistent results despite intensive sampling, an average value over several analysis samples can be taken as a last resort. If, in the second step, several such "mean value measurements" are again compared with each other and these do not deviate significantly from each other, the averaged result can be regarded as representative.

Figure 4: Combination of Vibratory Feeder LABORETTE 24 and Rotary Cone Sample Divider LABORTTE 27 for sample division (left) and ANALYSETTE 22 NeXT for analysis of particle size distribution by static light scattering (right).
Competent sampling leads to reliable particle measurement results
This article demonstrates the need for judicious sampling for representative, reproducible analytical results. For methods such as static light scattering, where a small amount of sample material is sufficient for analytics, sampling is a crucial analytical step to generate comparable and trustworthy results. In this context, it is important for the evaluation of a measurement to describe the exact process of sampling in order to assess the quality of the measurement. It remains to be noted that even the best sampling only provides a statistical certainty for the "true" result, nevertheless, by adept sampling the true result can be approached more closely and the reliability can be increased enormously.
-
Download the FRITSCH-report as PDF file